Why the Desired Effect is not Achieved After Using AAV?
Adeno-associated virus (AAV) is a small non-enveloped virus, belonging to the family Parvoviridae. It was first discovered in 1965 from contaminants of adenovirus isolates. It has an icosahedral structure on the outside and a diameter of about 26 nm. Its capsid protein is composed of It is composed of three proteins: VP1, VP2 and VP3. The genome of AAV is a single-stranded linear DNA, approximately 4700 bp, including two upstream and downstream open reading frames (ORFs): Rep and Cap. They are located between 2 T-shaped inverted terminal repeats (ITRs) each consisting of 145 nucleotides. The role of ITR is to serve as the origin of viral replication and packaging signal. The Rep gene is involvedin viral replication and integration, encoding viral replication proteins, and the Cap gene is responsible for encoding the three viral capsid proteins. The natural wild-type adeno-associated virus that exists in nature has Rep and Cap genes on its genome, while the experimental AAV vector is an artificially modified plasmid based on wild adeno-associated virus and does not have Rep and Cap genes on its genome. Therefore, also called recombinant adeno-associated virus (rAAV). Unless otherwise specified, the abbreviation AAV generally refers to the modified AAV vector.
There are many serotypes of AVV. Currently, 12 AAV serotypes have been isolated from humans and monkeys. Different AAV serotypes have different capsid protein spatial structures, sequences and tissue specificities. Therefore, the cell surface receptors they recognize and bind to are also very different. , which also results in different tissue types, cell types and infection efficiencies transfected by different serotypes. In the process of applying AAV virus, it is necessary to select the AAV virus of the corresponding serotype according to different tissues and organs.
Because adeno-associated virus has the advantages of wide host range, high safety, low immunogenicity, stable expression and stable physical properties, it has been widely used in basic research and clinical trials, and adeno-associated virus vectors have become one of the most commonly used gene therapy vectors in the world.
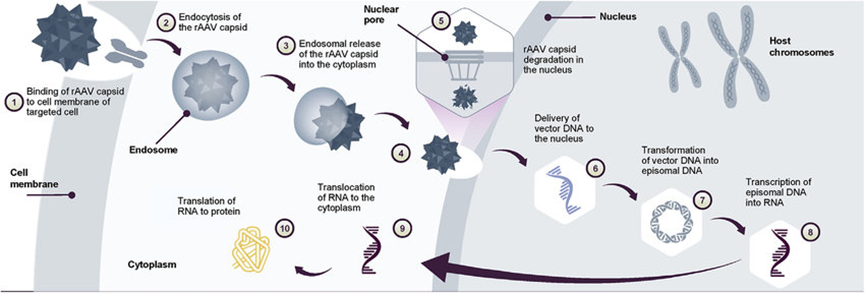
Figure 1. AAV gene transfer therapy mechanism of action Following gene transfer therapy administration.
After injecting AAV into animals, the desired effect is not achieved, or the overexpression or knockdown of the target gene cannot even be detected. What are the reasons?
This issue involves many possibilities, which need to be eliminated one by one and comprehensive consideration of various influencing factors:
Virus issues: Whether the size of the foreign gene sequence is within the packaging capacity of AAV. The packaging capacity of AAV usually does not exceed 4.7kb. After removing components such as promoters, fluorescent tags, and protein tags, the actual capacity of foreign genes is generally within Under 2kb. Furthermore, whether the virus titer meets the requirements, it is recommended that viruses stored for more than half a year should be re-measured before use. Also, the storage method must be appropriate and avoid repeated freezing and thawing. If you plan to use it within a week, you can store it at 4°C. For long-term storage, you need to aliquot and store it at -80°C.
Tissue/cell specificity issues: AAV has serotypes with different tissue tropisms and can carry specific promoters. Different tissue sites may have different injection methods and injection volumes. These are all factors in AAV infection efficiency. and important influencing factors of expression characteristics.
Detection time issue: Whether the detection time point is during the peak period of AAV expression, testing too early or too late may not achieve the desired results.
Background expression level of the target gene: Before constructing AAV to regulate gene expression, has the background expression level of the target gene in the target tissue or cells been detected? Higher background expression will cause overexpression, and lower background expression will cause interference. The regulatory effect may not be observed.
Problems with the method of sampling: whether the time, method, and storage conditions for collecting animal tissues or cells are appropriate, such as whether to choose paraffin sections or frozen sections; if you want to specifically detect a certain type of cells, you can use flow sorting and other methods to separate the target cells from Separate it from the tissue and then test it.
Detection method issues: Testing the expression level of the target gene usually involves qPCR (mRNA level) and WB (protein level). One issue to consider is whether AAV is expected to infect the entire tissue or a certain cell in the tissue. The latter It is necessary to isolate the target cells and then detect the expression level. In addition, the quality of the reagents, primers, and antibodies used in the detection should also be considered. If AAV has a fluorescent label, fluorescence can also be directly observed through in vivo imaging and tissue frozen sections to reflect the infection efficiency. It is recommended to use different methods and levels to detect AAV infection efficiency and target gene regulation effects.
Influence of regulatory factors of tissue cells themselves: In fact, in most cases, there is not a linear relationship between mRNA and protein expression levels. This process involves factors such as translation regulation, protein modification, and protein half-life. In addition, there may be a tendency for compensatory expression to increase or decrease, leading to undetectable significant changes in the protein.
Other issues: Whether the selection and health status of experimental animals meet the needs, whether the experimental operation technology is proficient, and the correct use of instruments are also keys to the success or failure of the experiment.





Share